TransCon™: A powerful technology platform central to our innovative approach
At the core of Ascendis Pharma is a powerful, flexible technology platform that uses advanced knowledge of chemistry to overcome challenges of developing new therapeutics. When applied using known biology, this technology platform helps us address unmet patient needs in unprecedented ways.
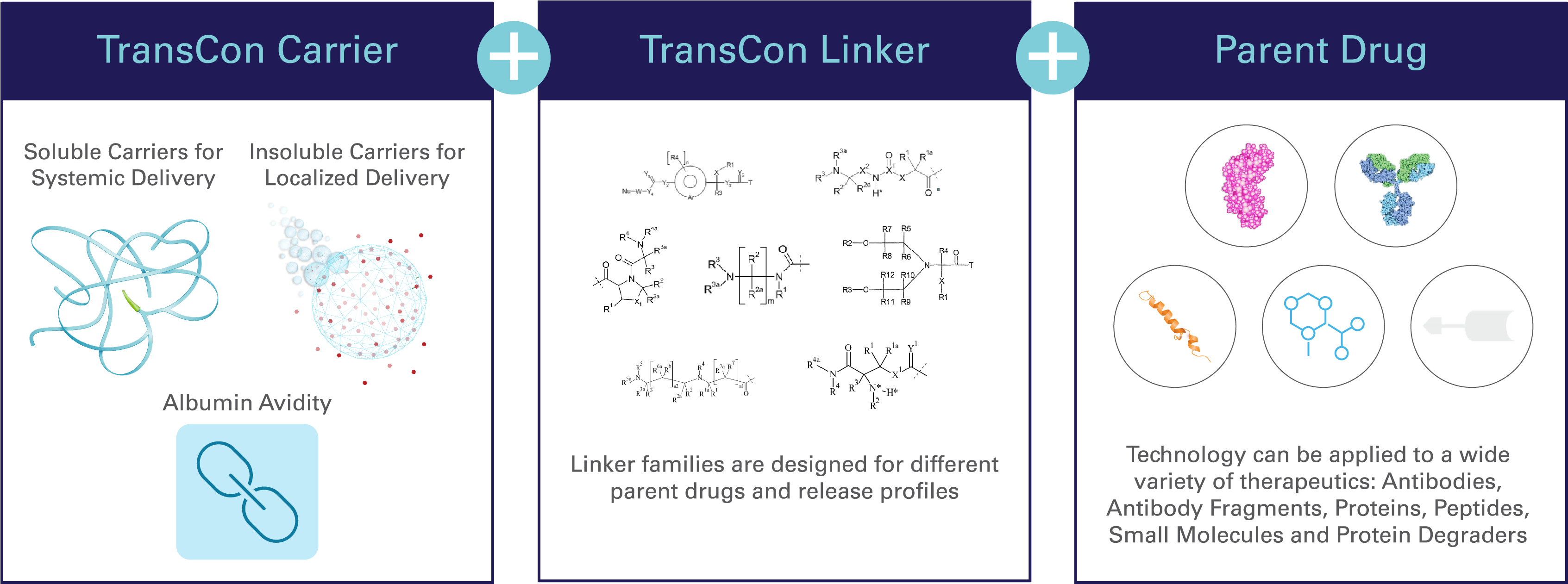
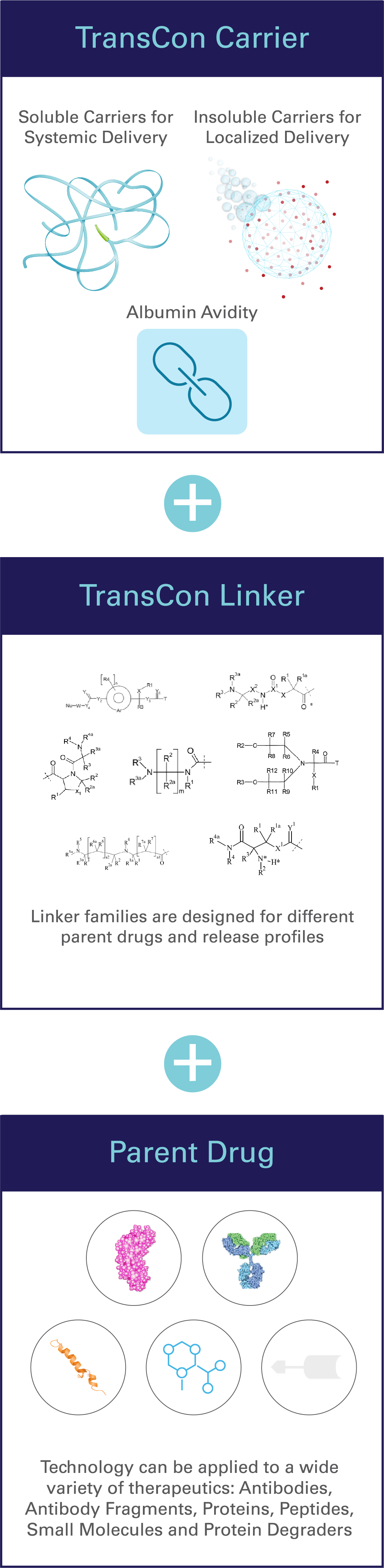
TransCon = transient conjugation
TransCon refers to “transient conjugation,” or our unique ability to temporarily (transiently) link an inert carrier to a parent drug with known biology.
Depending on the carrier used, TransCon prodrugs can be designed to act systemically (throughout the body) or locally (e.g. intratumorally), focused on meeting a specific therapeutic goal. TransCon is widely applicable to proteins, peptides or small molecules in multiple therapeutic areas1-3.
- Karpf, D.B., Pihl, S., Mourya, S., Mortensen, E., Kovoor, E., Markova, D. and Leff, J.A. (2020). A Randomized Double‐Blind Placebo‐Controlled First‐In‐Human Phase 1 Trial of TransCon PTH in Healthy Adults. Journal of Bone and Mineral Research, 35(8), pp.1430–1440. doi:https://doi.org/10.1002/jbmr.4016.
- Khan, A.A., Rejnmark, L., Rubin, M., Schwarz, P., Vokes, T., Clarke, B., Ahmed, I., Hofbauer, L., Marcocci, C., Pagotto, U., Palermo, A., Eriksen, E., Brod, M., Markova, D., Smith, A., Pihl, S., Mourya, S., Karpf, D.B. and Shu, A.D. (2021). PaTH Forward: a Randomized, Double-Blind, Placebo-Controlled Phase 2 Trial of TransCon PTH in Adult Hypoparathyroidism. The Journal of Clinical Endocrinology & Metabolism. doi:https://doi.org/10.1210/clinem/dgab577.
- Holten‐Andersen, L., Pihl, S., Rasmussen, C.E., Zettler, J., Maitro, G., Baron, J., Heinig, S., Hoffmann, E., Wegge, T., Krusch, M., Faltinger, F., Killian, S., Sprogoe, K., Karpf, D.B., Breinholt, V.M. and Cleemann, F. (2019). Design and Preclinical Development of TransCon PTH, an Investigational Sustained‐Release PTH Replacement Therapy for Hypoparathyroidism. Journal of Bone and Mineral Research, 34(11), pp.2075–2086. doi:https://doi.org/10.1002/jbmr.3824.